How Many Electrons Can the First Orbital Hold
Electronic Orbitals
- Page ID
- 1650
An cantlet is composed of a nucleus containing neutrons and protons with electrons dispersed throughout the remaining space. Electrons, withal, are not simply floating within the atom; instead, they are fixed inside electronic orbitals. Electronic orbitals are regions within the cantlet in which electrons have the highest probability of being plant.
Quantum Numbers describing Electronic Orbitals
There are multiple orbitals within an cantlet. Each has its ain specific energy level and backdrop. Because each orbital is unlike, they are assigned specific quantum numbers: 1s, 2s, 2p 3s, 3p,4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. The numbers, (n=i,two,3, etc.) are called principal quantum numbers and can only be positive numbers. The messages (south,p,d,f) represent the orbital angular momentum quantum number (ℓ) and the orbital athwart momentum quantum number may be 0 or a positive number, but tin never exist greater than n-1. Each letter is paired with a specific ℓ value:
s: subshell = 0
p: subshell = 1
d: subshell = 2
f: subshell = three
An orbital is also described by its magnetic quantum number (m ℓ ). The magnetic quantum number tin can range from –ℓ to +ℓ. This number indicates how many orbitals there are and thus how many electrons can reside in each atom.
Orbitals that take the aforementioned or identical energy levels are referred to equally degenerate. An example is the 2p orbital: 2px has the same free energy level as 2py. This concept becomes more of import when dealing with molecular orbitals. The Pauli exclusion principle states that no two electrons can have the same exact orbital configuration; in other words, the aforementioned quantum numbers. However, the electron can exist in spin upwardly (1000due south = +1/2) or with spin down (yards = -1/2) configurations. This ways that the s orbital can contain up to two electrons, the p orbital tin contain upwards to six electrons, the d orbital can comprise up to x electrons, and the f orbital can contain up to 14 electrons.
| s subshell | p subshell | d subshell | f subshell |
|---|---|---|---|
| ℓ = 0 | ℓ = 1 | ℓ = 2 | ℓ = three |
| kℓ = 0 | mℓ= -1, 0, +1 | mℓ= -2, -1, 0, +1, +two | thouℓ= -three, -2, -1, 0, +1, +2, +iii |
| One southward orbital | Three p orbitals | V d orbitals | Vii f orbitals |
| 2 s orbital electrons | 6 p orbital electrons | ten d orbital electrons | 14 f orbital electrons |
Visualizing Electron Orbitals
As discussed in the previous section, the magnetic quantum number (thousandl) can range from –l to +50. The number of possible values is the number of lobes (orbitals) at that place are in the south, p, d, and f subshells. As shown in Table 1, the s subshell has 1 lobe, the p subshell has three lobes, the d subshell has 5 lobes, and the f subshell has seven lobes. Each of these lobes is labeled differently and is named depending on which aeroplane the lobe is resting in. If the lobe lies along the x plane, then it is labeled with an x, equally in 2px. If the lobe lies along the xy airplane, so it is labeled with a xy such every bit dxy. Electrons are found within the lobes. The airplane (or planes) that the orbitals do not fill are chosen nodes. These are regions in which there is a 0 probability density of finding electrons. For example, in the dyx orbital, there are nodes on planes xz and yz. This tin can be seen in Figure \(\PageIndex{ane}\).
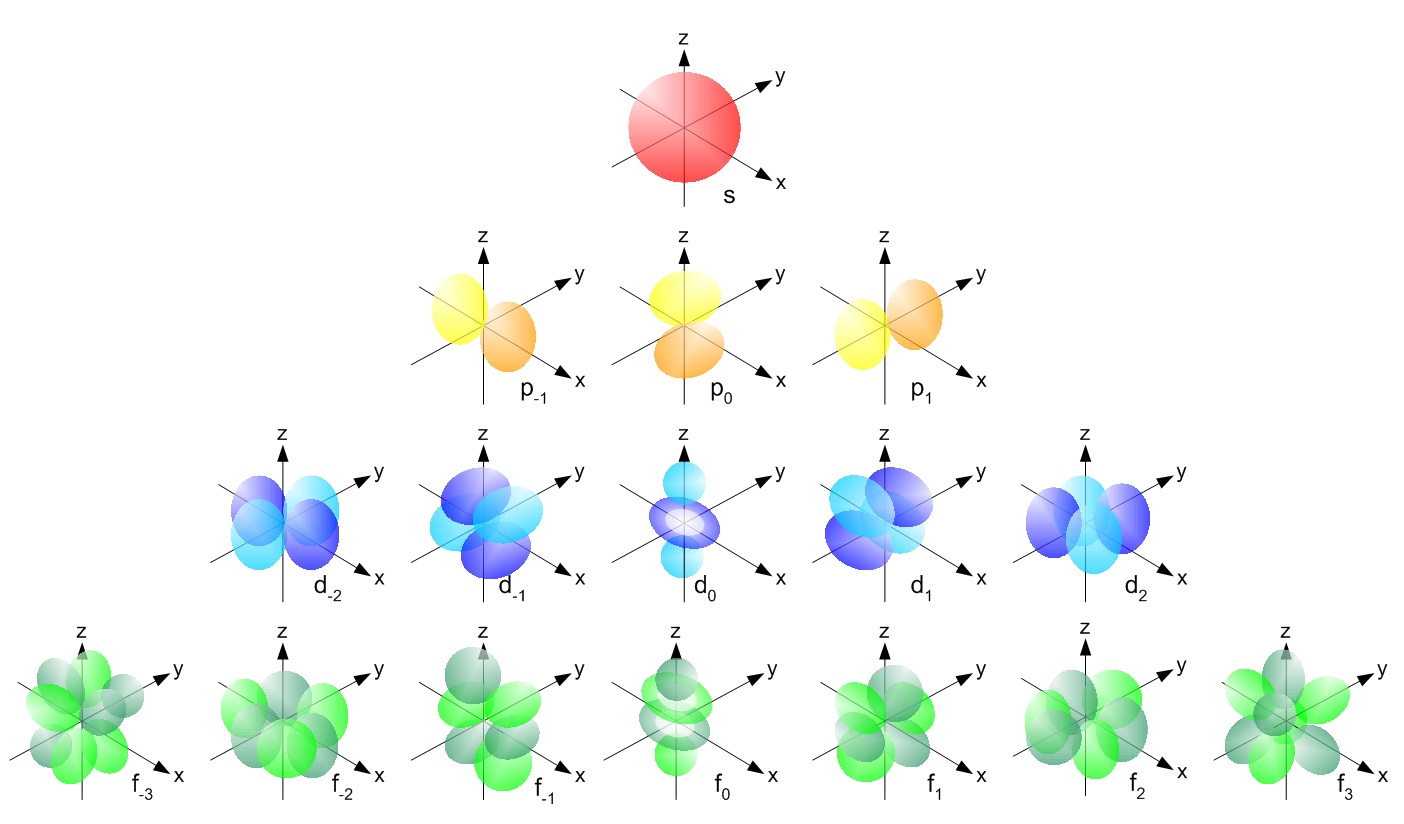
Radial and Angular Nodes
In that location are ii types of nodes, angular and radial nodes. Angular nodes are typically apartment airplane (at fixed angles), like those in the diagram above. The ℓ quantum number determines the number of angular nodes in an orbital. R adial nodes are spheres (at stock-still radius) that occurs as the principal quantum number increases. The total nodes of an orbital is the sum of angular and radial nodes and is given in terms of the \(n\) and \(l\) quantum number past the post-obit equation:
\[ Due north = north-l -one\]
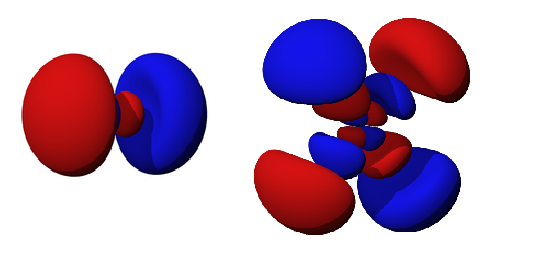
For example, determine the nodes in the 3pz orbital, given that north = 3 and ℓ = one (considering it is a p orbital). The full number of nodes present in this orbital is equal to n-1. In this example, 3-i=2, so there are 2 total nodes. The breakthrough number ℓ determines the number of angular nodes; there is one angular node, specifically on the xy airplane because this is a pz orbital. Considering there is one node left, there must be ane radial node. To sum upward, the 3pz orbital has 2 nodes: 1 athwart node and 1 radial node. This is demonstrated in Figure 2.
Another case is the 5dxy orbital. There are 4 nodes total (v-1=4) and there are two athwart nodes (d orbital has a quantum number ℓ=ii) on the xz and zy planes. This means there at that place must be two radial nodes. The number of radial and athwart nodes can merely be calculated if the master quantum number, type of orbital (s,p,d,f), and the aeroplane that the orbital is resting on (x,y,z, xy, etc.) are known.
Electron Configuration inside an Orbital
We can think of an atom like a hotel. The nucleus is the lobby where the protons and neutrons are, and in the floors to a higher place, nosotros find the rooms (orbitals) with the electrons. The principal quantum number is the flooring number, the subshell blazon lets u.s. know what type of room it is (s being a cupboard, p beingness a single room, d having 2 adjoining rooms, and f being a suit with three rooms) , the magnetic quantum number lets us know how many beds there are in the room, and two electrons can sleep in i bed (this is considering each has a unlike spin; -ane/two and 1/two). For example, on the first floor nosotros have the due south orbital. The south orbital is a closet and has one bed in it so the get-go floor can agree a total of ii electrons. The second floor has the room styles s and p. The s is a cupboard with one bed equally nosotros know and the p room is a single with three beds in it so the 2d floor can hold a full of eight electrons.
Each orbital, every bit previously mentioned, has its own energy level associated to information technology. The everyman free energy level electron orbitals are filled starting time and if at that place are more than electrons after the lowest energy level is filled, they move to the next orbital. The society of the electron orbital energy levels, starting from least to greatest, is every bit follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
Since electrons all accept the same charge, they stay every bit far away as possible because of repulsion. Then, if there are open orbitals in the same energy level, the electrons will fill each orbital singly before filling the orbital with two electrons. For example, the 2p shell has iii p orbitals. If there are more electrons after the 1s, and 2s orbitals take been filled, each p orbital will be filled with i electron first before 2 electrons endeavor to reside in the same p orbital. This is known every bit Hund's rule.
The style electrons motility from i orbital to the next is very similar to walking up a flight of stairs. When walking up stairs, you identify i foot on the beginning stair and then another foot on the second stair. At any point in time, you can either stand with both feet on the first stair, or on the second stair simply it is impossible to stand in between the two stairs. This is the way electrons motility from one electron orbital to the next. Electrons tin can either jump to a higher energy level by absorbing, or gaining free energy, or driblet to a lower energy level by emitting, or losing free energy. However, electrons will never exist found in between two orbitals.
Problems
- Which orbital would the electrons fill starting time? The 2s or 2p orbital?
- How many d orbitals are there in the d subshell?
- How many electrons tin can the p orbital hold?
- Determine the number of angular and radial nodes of a 4f orbital.
- What is the shape of an orbital with 4 radial nodes and one athwart node in the xy airplane?
Solutions
- The 2s orbital would exist filled before the 2p orbital considering orbitals that are lower in free energy are filled first. The 2s orbital is lower in energy than the 2p orbital.
- In that location are 5 d orbitals in the d subshell.
- A p orbital can hold six electrons.
- Based off of the given data, n=4 and ℓ=3. Thus, in that location are 3 athwart nodes present. The total number of nodes in this orbital is: 4-1=3, which ways at that place are no radial nodes nowadays.
- 1 athwart node ways ℓ=ane which tells usa that nosotros accept a p subshell, specifically the pz orbital because the angular node is on the xy airplane. The total number of nodes in this orbital is: 4 radial nodes +one angular node=five nodes. To detect north, solve the equation: nodes=n-1; in this case, 5=n-one, and then n=half-dozen. This gives u.s.a. a: 6pz orbital
References
- General Chemistry Principles & Modern Applications. ninth ed. New Jersey: Pearson Education, Inc, 2007. Impress.
- A new Dictionary of Chemical science. 3rd ed. Great Britian: Longman Dark-green & Co., 1961. Impress.
- General Chemistry. Usa: Linus Pauling, 1947. Print.
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals
0 Response to "How Many Electrons Can the First Orbital Hold"
Post a Comment